Abstract
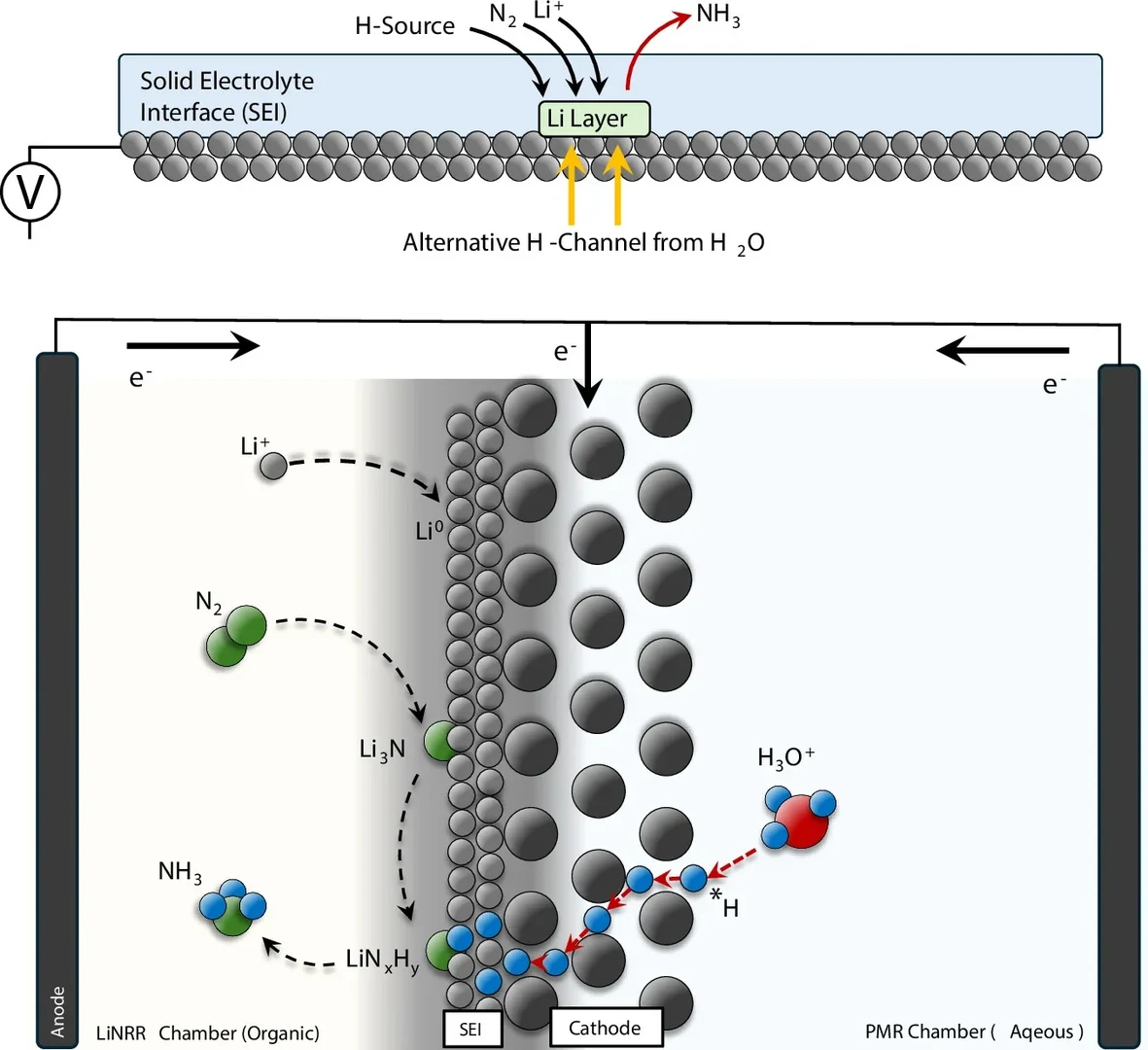
Lithium-mediated N2 reduction reaction (LiNRR) is regarded as the most robust route towards electrifying NH3 synthesis. However, in this reaction geometry, hydrogen atoms typically supplied through electrolyte degradation, via H2 oxidation or a combination of both, hampering the efficiency of the process. In this work we provide an alternative H-source by merging a Pd Membrane reactor (PMR) with a LiNRR reactor in a unique dual-reactor setup. Specifically, use a Pd membrane that extracts H atoms directly from H2O and transfers them across the membrane to an electrodeposited Li layer operating under non-aqueous LiNRR conditions. We show that these H2O-derived H-atoms are used directly to synthesize NH3 in the presence of N2 and electrodeposited Li, thereby opening orthogonal reaction pathways within the metal-mediated nitrogen reduction concept.