Research Interest of the Bismuto Group
© A. Bismuto
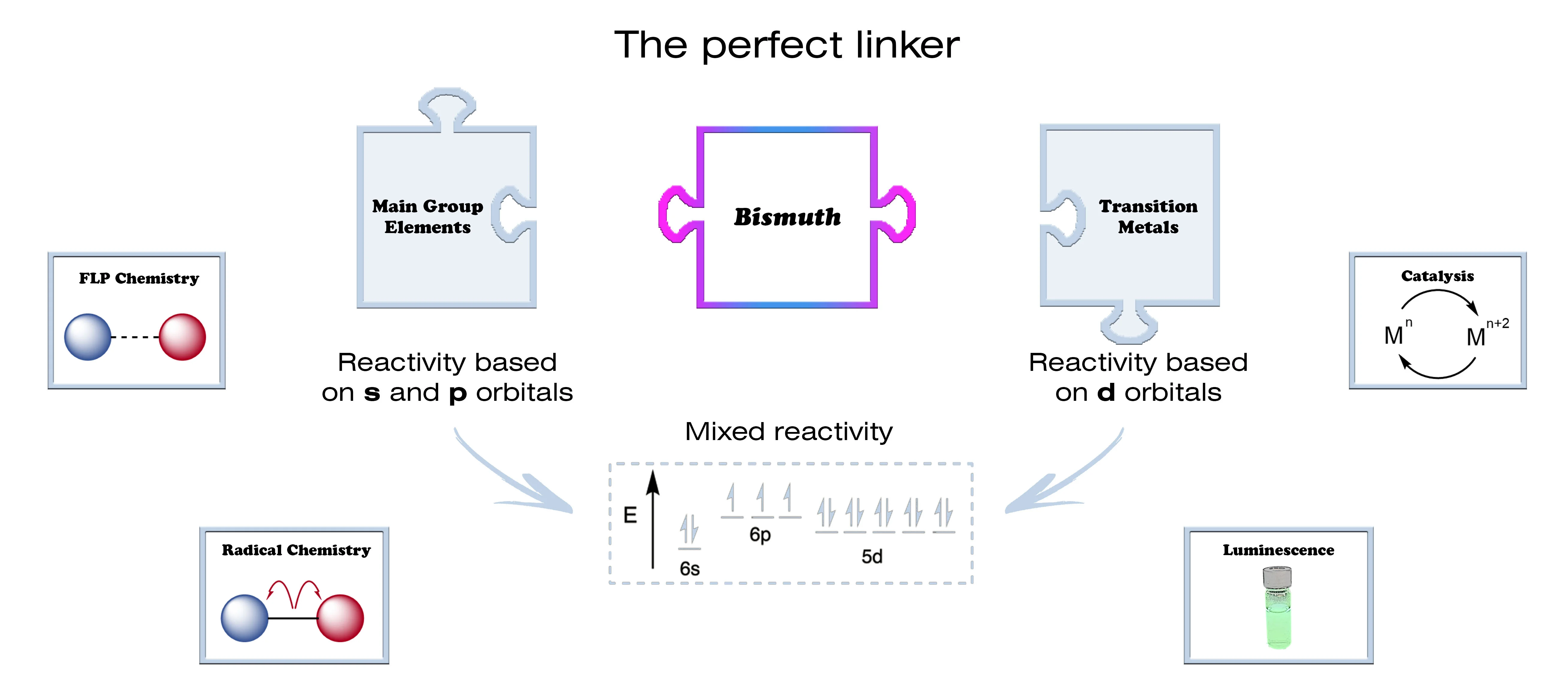
Bismuth is the heaviest stable element in the periodic table, which accounts for the unusual electronic behaviours observed. Due to the large number of electrons available, the energy gap between its orbitals is considerably lower than that of lighter main-group elements. This allows bismuth to span several oxidation states, mimicking transition metal reactivity. In addition, the ability of bismuth to expand its valence shell (hypervalency) can lead to materials with unusual electronic properties. Thus, bismuth shows a great potential to merge the benefits of both main-group and transition metal elements, so enabling new applications in a broad range of fields including catalysis and optoelectronics.


