Welcome to the Homepage of
Prof. Dr. A. C. Filippou
Inorganic Molecular Chemistry, Organometallic Chemistry
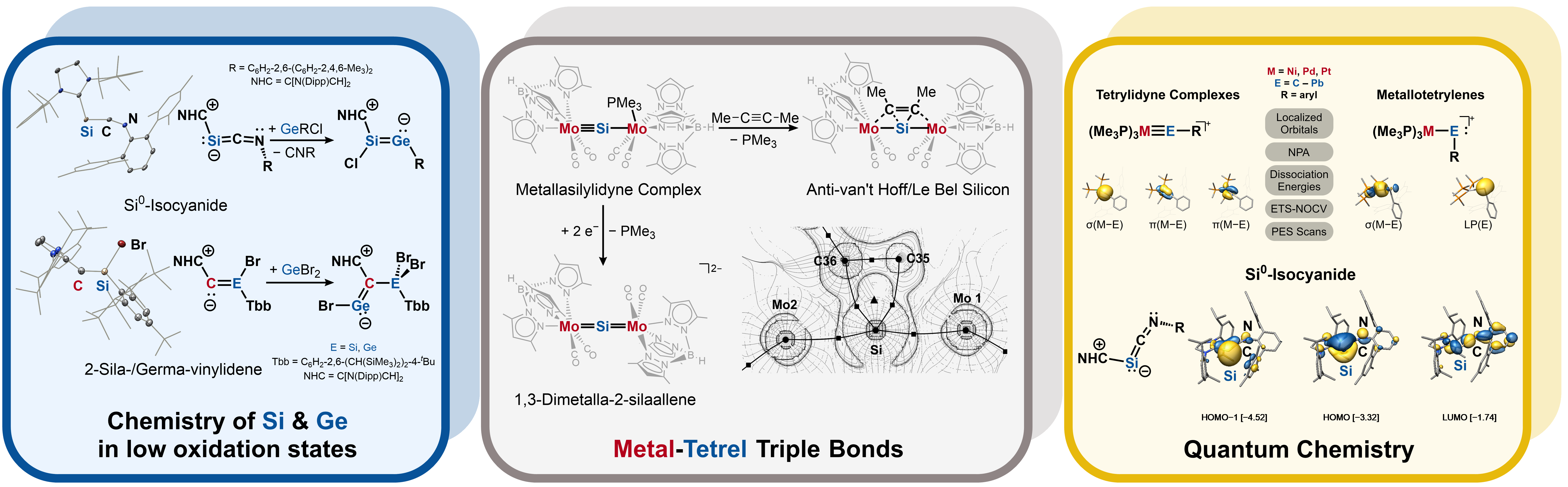
Research areas
Research in the Filippou group focuses on the areas of advanced molecular transition metal and main group element chemistry, combining the potential of both fields to create new compounds with unprecedented bonding motifs and reactivity. The focus lies on isolating and characterizing highly reactive closed- and open-shell molecules containing silicon, germanium, and transition metals in novel bonding environments, and exploring the ability of these compounds to generate new materials in stochiometric or catalytic reactions. Advanced synthetic methods and state-of-the-art analytical and computational techniques are used to achieve these goals.
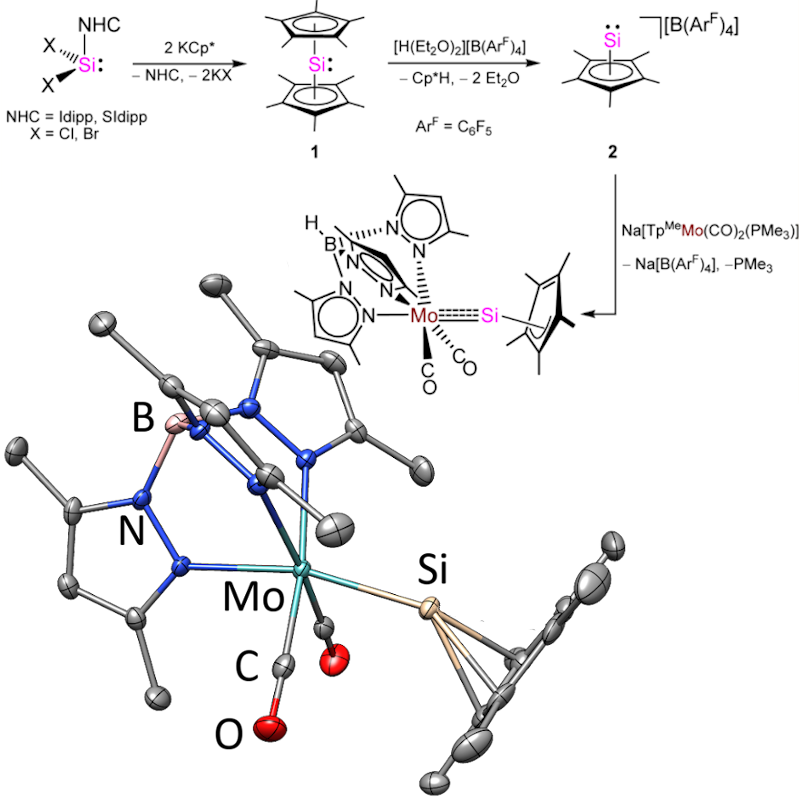
Triple bonds of Si - Pb with transition metals
We have been studying the chemistry of tetrelylidine complexes of the general formula LnM≡ER, where M is a transition metal, Ln is a ligand sphere, E = Si - Pb, and R is an organyl substituent, for over 20 years.
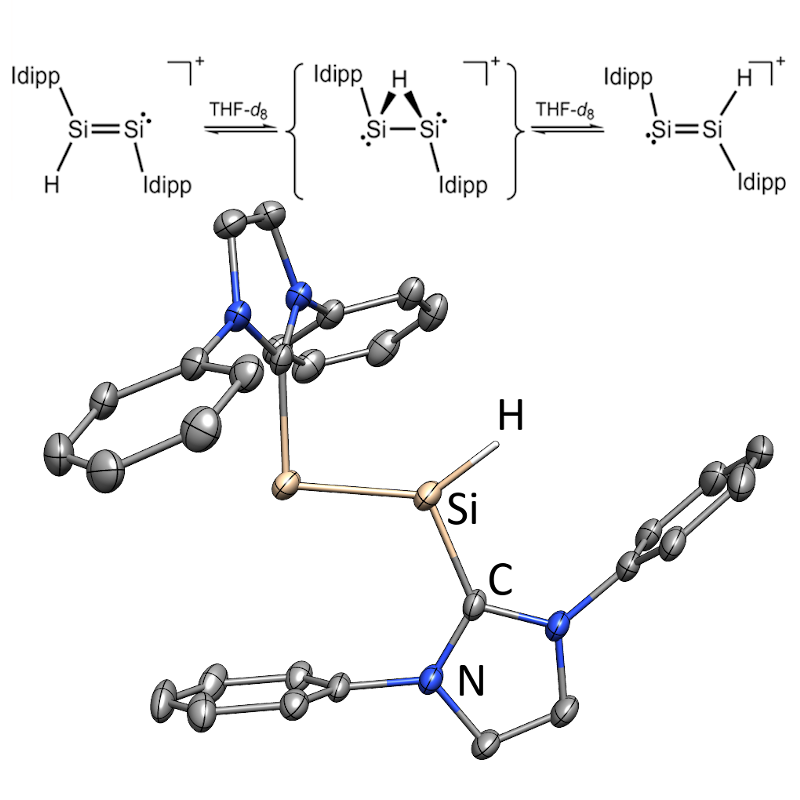
Low-valent Silicon Chemistry
Due to its abundance, silicon as a ubiquitous component in technologically important materials is an element with high future potential. In our research group, we aim to tame and explore novel, highly reactive silicon compounds.
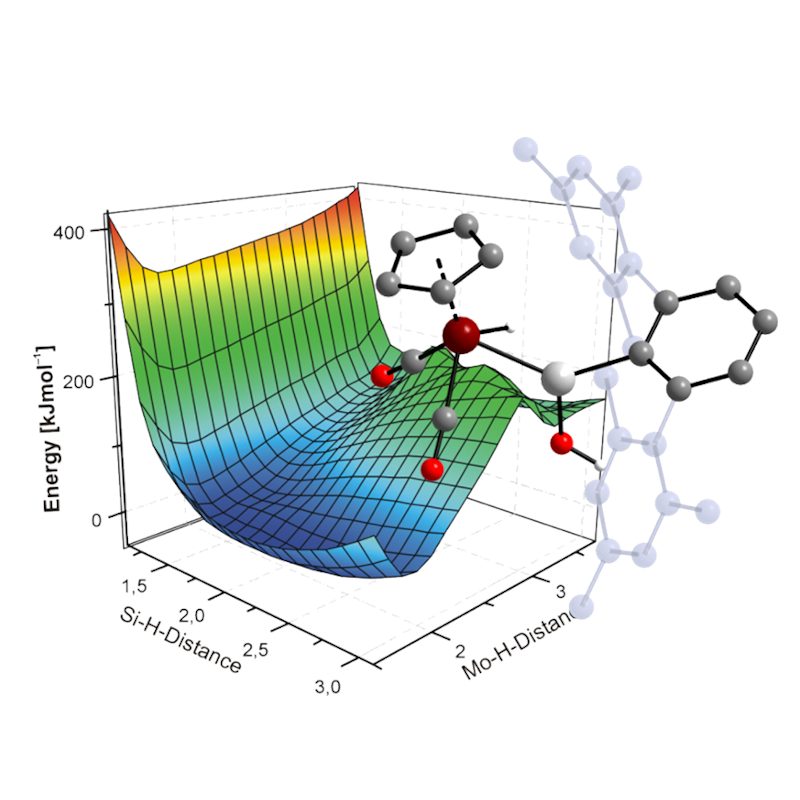
Quantum Chemistry
Our research is supported by state-of-the-art computational chemistry methods to gain deeper insight into the properties of the compounds we study and to predict and/or explain reactivities.


