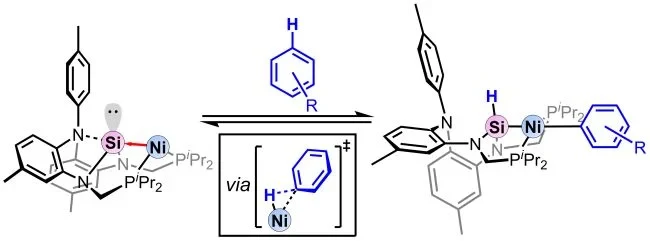
The publication, entitled “Reversible C-H Bond Activation of Unactivated Arenes by a Nickel-Silylene Complex,” has been released as a JACS communication. In the paper, Gomm et al. describe a nickel-silylene complex that can reversibly activate aryl C–H bonds in benzene and other unactivated aromatics. The work was led by the Lu group in collaboration with the Grimme group. First-author Leon Gomm (AK Lu) and Dr. Hui Zhu (AK Grimme) worked jointly to elucidate the mechanism.
ABSTRACT: A nickel-silylene complex is shown to reversibly activate benzene via C−H bond activation at ambient temperature. The benzene C−H bond formally adds across the Ni−Si core to form a nickel-silyl complex with an H−Si−Ni−Ph linkage bearing a highly covalent Ni−Si bond (2.2363(6) A). Dissolution of this product in neat arene solvent, e.g., toluene, trifluorotoluene, or anisole, resulted in the complete conversion to the aryl C−H activated product of the solvent and, thereby, demonstrates the facile reversibility of C−H bond activation across the Ni−Si core. The various arene C−H bond activations by the reactive Ni(0)-silylene species were investigated using density functional theory calculations. In all cases, the arene initially forms an η2-adduct to Ni(0), and then C−H activation proceeds via a concerted oxidative addition at only the Ni center. The Si center assists C−H bond activation by sharing the redox burden with Ni while stabilizing the resultant hydride.
To the publication: https://doi.org/10.1021/jacs.5c10922